Introduction
In the world of chemical reactions and organic compounds, understanding the interaction between specific molecules can unlock massive potential in both industrial and academic settings. One such intriguing compound set is captured by the expression “hcooch ch2 h2o.” While this might initially appear cryptic, it refers to the hydrolysis process involving methyl formate and water. This article delves deep into the meaning, chemical behavior, reactions, applications, and significance of “hcooch ch2 h2o” in a clear, engaging, and trustworthy manner.
What Is Meant by ‘HCOOCH CH2 H2O’?
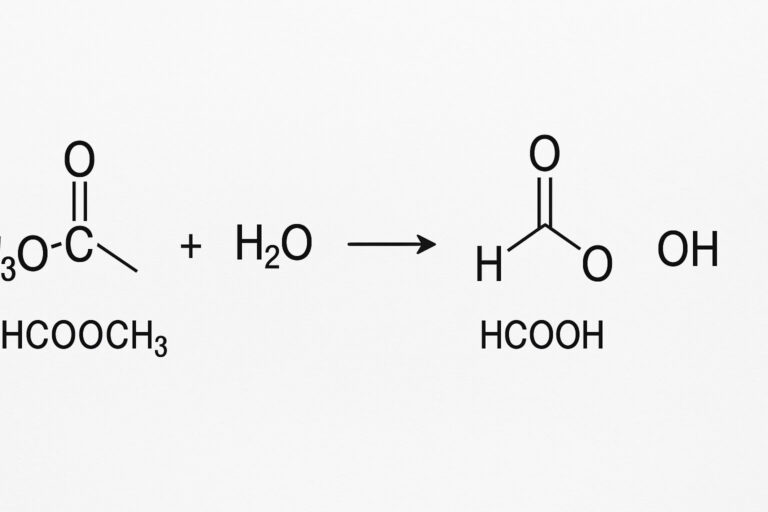
The term “hcooch ch2 h2o” is best interpreted as a shorthand notation for the reaction involving methyl formate (HCOOCH3) and water (H2O). When methyl formate reacts with water, a hydrolysis reaction occurs. This leads to the formation of formic acid (HCOOH) and methanol (CH3OH). The reaction can be summarized as:
HCOOCH3 + H2O → HCOOH + CH3OH
Although the notation “CH2” appears in the keyword, it is likely a confusion or misrepresentation of “CH3” in methyl formate.
Breaking Down the Components
1. Methyl Formate (HCOOCH3)
Methyl formate is a colorless liquid with a pleasant odor, commonly used as a solvent and intermediate in various chemical processes. It is an ester derived from formic acid and methanol.
2. Water (H2O)
Water is a universal solvent, often involved in hydrolysis reactions where it breaks down chemical compounds into their simpler components.
3. Formic Acid (HCOOH)
A product of the hydrolysis reaction, formic acid is known for its use in agriculture, leather processing, and as a preservative.
4. Methanol (CH3OH)
Methanol is a volatile, flammable liquid widely used in industrial applications, including fuel, antifreeze, and as a solvent.
The Hydrolysis Reaction: How It Works
The hydrolysis of methyl formate by water is an acid-catalyzed process. In simpler terms, water interacts with methyl formate under the influence of an acid catalyst, which facilitates the breaking of the ester bond in methyl formate, resulting in the formation of formic acid and methanol. This reaction is vital for understanding basic ester chemistry and has real-world implications.
Industrial and Practical Applications
1. Chemical Manufacturing
This reaction is used to produce formic acid and methanol in controlled environments. Both these chemicals are essential in various manufacturing sectors.
2. Solvent Production
Methanol produced via this reaction is used as a base chemical for other solvents and materials.
3. Agricultural Use
Formic acid, a product of this reaction, serves as a preservative and antibacterial agent in livestock feed.
4. Educational Demonstrations
The simplicity and clarity of this reaction make it an excellent example for teaching ester hydrolysis in academic labs.
Environmental Considerations
This reaction involves substances that are biodegradable, but they must still be handled with care. Methyl formate and methanol are flammable and potentially toxic, so industrial use requires proper safety protocols. Nevertheless, the overall environmental footprint is considered manageable when standard practices are followed.
Safety Measures
- Proper Ventilation: Both reactants and products should be handled in well-ventilated areas.
- Protective Equipment: Use of gloves, goggles, and protective clothing is essential.
- Storage Guidelines: These chemicals should be stored in cool, dry places, away from open flames or high heat.
Scientific Significance
The “hcooch ch2 h2o” reaction is more than just a simple chemical transformation. It exemplifies a class of reactions known as ester hydrolysis, which is a cornerstone concept in organic chemistry. Understanding how esters interact with water under different conditions helps chemists design and control numerous other reactions in both laboratory and industrial contexts.
Common Misconceptions
- “CH2” Confusion: Often, the component is mistakenly referred to as “CH2” instead of “CH3.” In this reaction, methyl formate contains a “CH3” group.
- Not a Biological Reaction: Although it involves water, this reaction is not typically found in biological systems.
- Not Reversible in Practice: While theoretically reversible, in industrial practice, the hydrolysis is usually complete due to reaction conditions.
5 Frequently Asked Questions (FAQs)
Q1: What exactly does ‘hcooch ch2 h2o’ represent?
- It refers to the hydrolysis of methyl formate with water, resulting in formic acid and methanol. The proper notation should be “HCOOCH3 + H2O.”
Q2: Is this reaction used in real-life industries?
- Yes, the products of this reaction—formic acid and methanol—are widely used in industrial processes.
Q3: Is the reaction safe?
- With proper safety protocols, including ventilation and protective gear, the reaction is safe to perform in labs and industrial settings.
Q4: Why is it called hydrolysis?
- “Hydrolysis” comes from the Greek words for water (hydro) and separation (lysis), meaning a compound is broken down by water.
Q5: Can this reaction be done at home?
- Due to the chemicals involved, including flammable and toxic substances, this reaction is not suitable for home experiments.
Conclusion
The reaction represented by “hcooch ch2 h2o” may seem puzzling at first glance, but it is a fundamental example of ester hydrolysis in chemistry. Understanding this transformation not only deepens one’s knowledge of organic chemistry but also opens the door to appreciating the vast industrial applications of formic acid and methanol. Whether for educational purposes or industrial synthesis, the principles behind this reaction are both important and highly relevant. As with all chemical processes, knowledge, precision, and safety are key to harnessing its full potential read also more. www thes oundstour.com